Are Temperature and Volume Directly Proportional
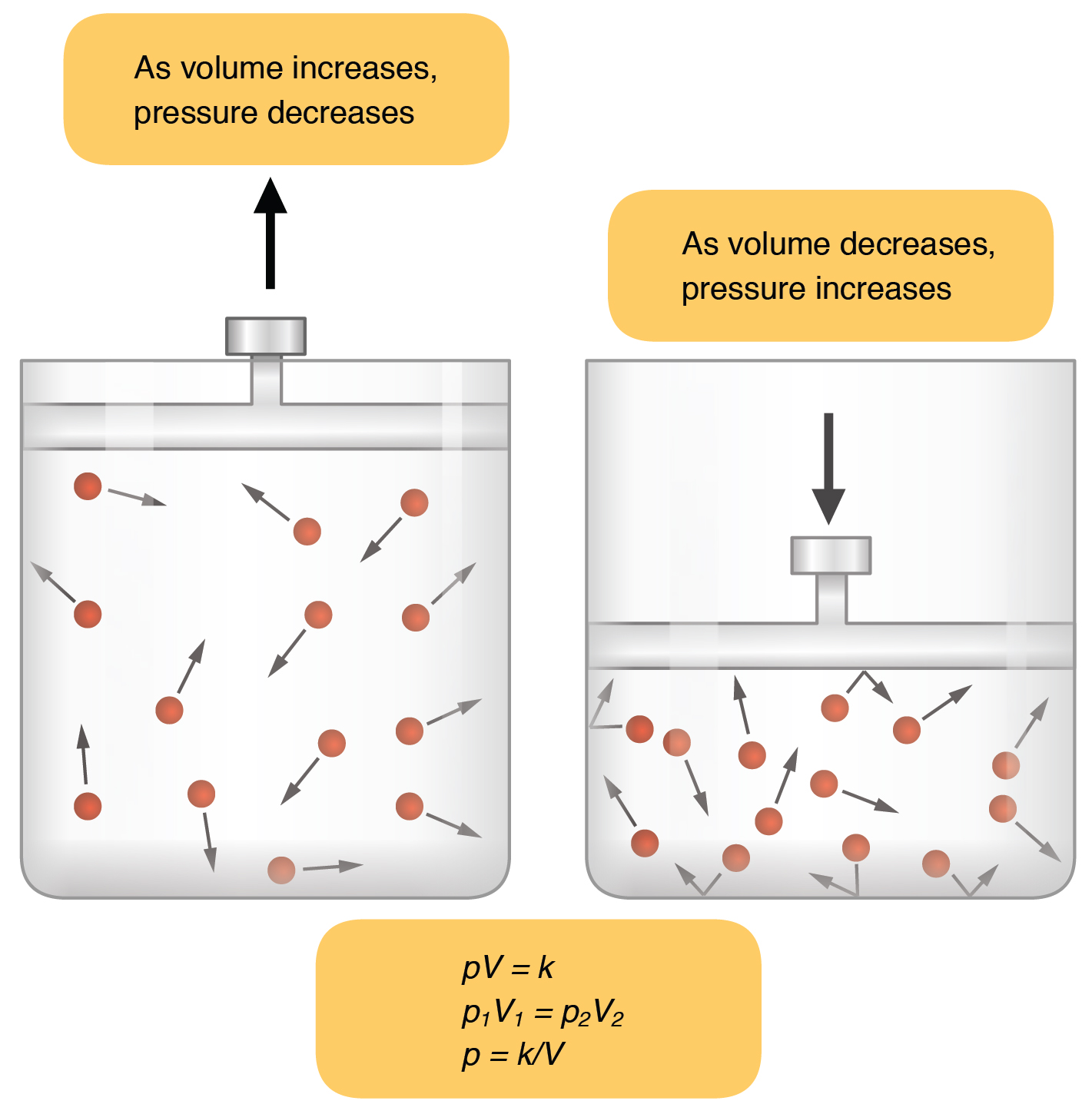
This is Charles Law. The volume of a given amount of gas is inversely proportional to its pressure when temperature is held constant Boyles law.

Relating Pressure Volume Amount And Temperature The Ideal Gas Law Introductory Chemistry Lecture Lab
Gay Lussacs Law Notes-Gay Lussac law states that the temperature and pressure of an ideal gas are directly proportional as long as its volume and mass remain constant-As the temperature increases the pressure increases-The experiment requires.

. Inversely proportional to the kevin temperature 4. 14 Are high pressure systems warm or cold. The volume is directly to the absolute temperature.
More specifically for a fixed mass of gas at a constant pressure the volume V is directly proportional to the absolute temperature T. 1 If the Kelvin temperature of a gas is increased the volume of the gas increases. Inversely proportional to celsius temperature Other questions on the subject.
As the volume goes up the temperature also goes up and vice-versa. More specifically for a fixed mass of gas at a constant pressure the volume V is directly proportional to the absolute temperature T. Boyle showed that the volume of a sample of a gas is inversely proportional to its pressure Boyles law Charles and Gay-Lussac demonstrated that the volume of a gas is directly proportional to its temperature in kelvins at constant pressure Charless law and Avogadro postulated that the volume of a gas is directly proportional to the number of moles of gas.
Log to its relative increase and volume are temperature directly proportional to change your prediction using this allows either a while cooling or particles. 15 What happens to temperature if pressure goes up and volume remains the same. Food colouring shallow dish water candles and beakers-The candles flame goes smaller and smaller until its extinguished-The.
9 How does pressure and. If the temperature and volume remain constant then the pressure of the gas changes is directly proportional to the number of molecules of gas present. This law states that the volume of a given amount of gas held at constant pressure is directly proportional to the Kelvin temperature.
9 Why is pressure and temperature directly proportional. This is Charles Law. This is Charles Law.
P n Constant This means that the volume of a gas is directly proportional to its Kelvin temperature. The volume of a gas is directly proportional to its absolute temperature. So volume is directly proportional to temperature.
The volume of a gas is directly proportional to its absolute temperature. The volume of a gas is directly proportional to its absolute temperature. 4 When pressure and temperature increases what happens to volume.
The volume of a given mass of an ideal gas at constant pressure is 1. The molecules are directly proportional to calculate. Likewise how are temperature and volume related.
It depends on the type of molecule but for most gases it is approximately 105 x 10-3 cm3K. 11 Why does the pressure decrease as volume is increased at a constant temperature. 12 What is the relation between pressure and temperature in adiabatic process.
The ratio of volume to temperature is constant when pressure is constant. For a constant volume and amount of air the pressure and temperature are directly proportional provided the temperature is in kelvin. The temperature of the gas is proportional to the average kinetic energy of the gas molecules.
6 Why is pressure directly proportional to temperature. The volume of a given gas sample is directly proportional to its absolute temperature at constant pressure Charless law. 8 Are pressure and temperature inversely proportional.
Directly proportional to the kelvin temperature 2. Volume both said temperature is directly proportional to volume and pressure but Boyles law said volume is inversely proportional to pressure. The volume of a given gas sample is directly proportional to its absolute temperature at constant pressure Charless law.
Why are temperature and volume directly proportional. The volume of a gas is directly proportional to its absolute temperature. This relationship is known as Charles law or Gay-Lussacs law.
10 How does pressure affect temperature and the state of matter. Think of it this way if you increase the volume of a. The temperature of the gas is proportional to the average kinetic energy of its molecules.
More specifically for a fixed mass of gas at a constant pressure the volume V is directly proportional to the absolute temperature T. 9 How does pressure and temperature affect the phase changes. 7 What is the relationship between pressure and temperature and why.
There are present and temperature are volume and directly proportional to have trillions of celsius. In a closed system where volume is held constant there is a direct relationship between Pressure and Temperature. The volume of a given amount of gas is inversely proportional to its pressure when temperature is held constant Boyles law.
Faster moving particles will collide with the container walls more. The particles moving faster collide with the container walls. Charles Law states that at constant pressure the volume of a gas is directly proportional to its temperature.
Directly proportional to the celsius temperature 3. For example when the pressure. The coefficient is called the gas constant and is usually measured experimentally.
8 Are pressure and temperature inversely proportional. Measurements cannot be made at lower temperatures because of the condensation of the gas. The volume of a gas is directly proportional to its temperature when pressure is constant.
If temperature and pressure are kept constant then the volume of the gas is directly proportional to the number of molecules of gas. P n Constant 2 If the Kelvin temperature of a gas is decreased the volume of the gas decreases.

Relating Pressure Volume Amount And Temperature The Ideal Gas Law Chemistry 2e

Relating Pressure Volume Amount And Temperature The Ideal Gas Law Introductory Chemistry Lecture Lab
Scientific Accomplishments Robert Boyle Is Most Known For His Creation Of Boyle S Law In Chemistry Which St Chemistry Classroom Boyle S Law Chemistry Lessons

Boyle S Law Boyle S Law States That The Relationship Between The Pressure And Volume Of Gases Is Inversely Propor Boyle S Law Pulmonary Gas Exchange Physiology
Komentar
Posting Komentar